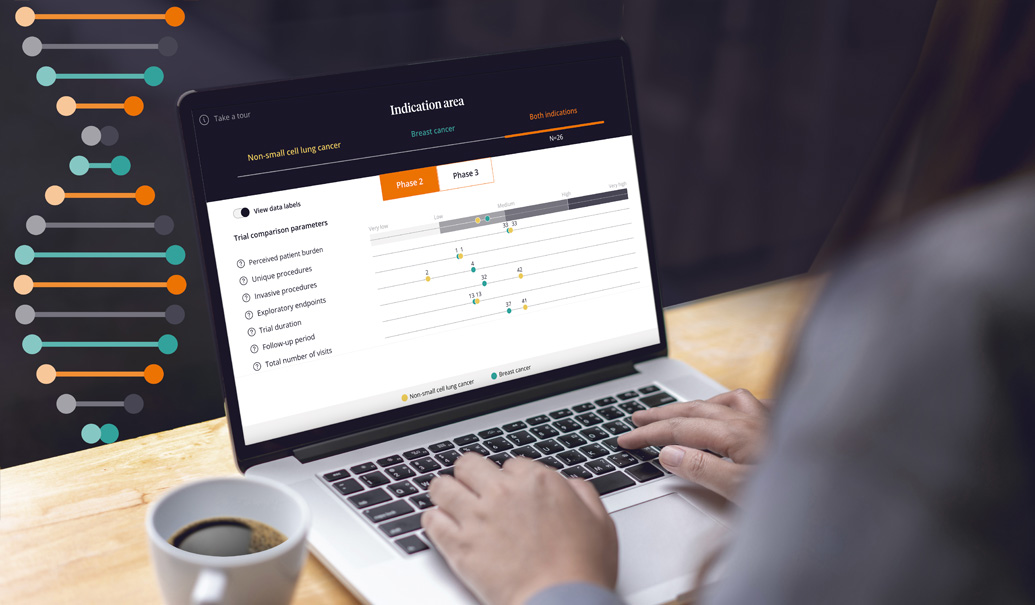
Despite the industry's attempts to make clinical trial design more inclusive and accessible, trial performance has remained stagnant. The proportion of clinical trials facing challenges with patient recruitment and retention has stayed the same since 2012, with 80% of trials reporting enrollment-related delays.
Decentralizing, digitizing and designing trials to be more patient-centric are some ways that the industry has effectively countered this trend, yet the complexities around the logistical and operational delivery of clinical trials continue to increase. The expanding focus on biomarker-directed oncology treatments, rare disease indications and personalized medicine could also increase operational burdens and lower engagement for patients, sites and sponsors.
To design more balanced trials, sponsors need a transparent and data-driven view of the complex elements that impact clinical trials. This interactive example explores the effect of parameters on trial performance, offering sponsors a new way to think about complexity as the industry strives to improve the experience for patients and sites.
The effects of trial complexity on clinical trial performance
Of course, overly complex trial design can lead to patient and site burden. We have seen a correlation between trial complexity—and its associated burden—with lower trial performance outcomes across a range of metrics. These include recruitment and retention, cycle times and quality measures such as deviations and amendments. The ZS patient burden analysis draws from an inclusive data set created by ZS and the Tufts Center for the Study of Drug Development (TCSDD), which hosts data from more than 3,000 patients worldwide. ZS’s protocol optimization capabilities can identify key risk areas at a procedural, visit and study level, all in an effort to optimize the site and patient experience.
This example highlights the power of parameters that define the complexity of clinical trials. Each day, we help sponsors more deeply understand how to optimize their trial designs at the indication, visit and procedure level, as well as other elements of burden and complexity. Many are already putting evaluations of protocol complexity to use, making changes such as:
- Removing exploratory endpoints that increase operational and data burden but are not critical for demonstrating efficacy or safety outcomes
- Establishing limits on the total time required for any study visit, because extended schedules—half- or full-day visits—contribute to lower patient enrollment and higher dropout rates
- Identifying which specific procedures or visits are most burdensome, and providing advance support to sites and patients
ZS clinical trial protocol optimization capabilities: How we examine complexity
As protocol complexity steadily increases, ZS’s protocol optimization capabilities offer quantifiable and repeatable methods to visualize and optimize trial design for improved outcomes and trial competitiveness. Our capabilities have helped sponsors mitigate downstream risks, while refining the trial experience for sites and patients, resulting in improved speed, cost and quality across the trial life cycle.
ZS’s clinical trial protocol optimization capabilities include advisory services, analytics offerings and the Trials.ai platform. We support sponsors and contract research organizations, helping them leverage data-driven approaches to compare the design elements of a clinical trial against industry benchmarks. Our work enables us to see outliers and drivers of complexity, which we look at from multiple dimensions to identify risks in a study’s design, including:
- Clinical: Number of endpoints and exploratory procedures, as well as deviations from standard of care
- Operational: Study placements and logistics, and number of visits and procedures
- Human-centric: Burden of participation for sites and patients, logistics, lifestyle and more
By visualizing complexity across these dimensions, teams can identify risks and make trade-offs to optimize the trial for all stakeholders, lower the burden of participation for sites and patients, reduce costs and time for sponsors and increase speed to outcomes. Our analysis is derived from a proprietary protocol database developed by ZS that integrates both ZS and publicly available sources to compare trials in a like-to-like manner and evaluate overall protocol complexity across therapy areas and phases. ZS updates this database on an ongoing basis.
If you want to better understand your trial’s complexity and burden, we can help. Contact ZS today.